Rusty Coolant/Coolant FAQ
#1
Registered User
Thread Starter
Rusty Coolant/Coolant FAQ
Recently I updated a response in a rusty coolant thread over on TN to include general info on coolant types, but also added a list of coolant capacities and the amount of coolant needed to make a 50% mix. Also a chart of freeze points for different coolant percents. I thought some folks here might find the info useful.
Original thead is here. The OP mentioned a radiator leak in addition to the rusty coolant.
--------------------------------------------------------------------------------------------------
Rusty coolant means coolant that lost its corrosion protection and became acidic. This will happen if not changed every two years, or if non-distilled water was used, or if different types of antifreeze were mixed (either by adding a different type or by not flushing thoroughly, including the heater core and plastic overflow tank), or by using one of the organic acid-based extended life coolants.
That's why the leak in the radiator.
The problem is that the corrosion is not limited to the radiator. The rust indicates the block has corroded, and, no doubt, the steel sealing rings on your head gasket(s) also. You'll have to keep a close eye out for signs of a failed head gasket.
I know because I made the same mistake with the same result except my pinhole leak was in one of the coolant pipes in that spaghetti cluster of vacuum and coolant pipes bolted to the front of the plenum on a 3vze. And not long after, my head gasket failed on cyl #1. When I removed the heads, I found the sealing ring there completely corroded with rust caused by the adjacent coolant gallery.
In my case I had replaced the toyota red coolant with prestone green, without flushing as thoroughly as I needed to, thus mixing two incompatible coolants which resulted in some of the silicates in the incoming coolant being neutralized by the phosphates in the old coolant. I think I also probably used tap water, further degrading the corrosion protection in the new coolant. A year later, like you, I opened the radiator cap one day to find the coolant completely rusty.
If you can let us know what type of coolant you used, how long it has been in there, whether it was mixed with any other type, and whether tap water was used, that will be useful info for the other folks who will read this.
I suppose its worth a try to patch your radiator, but if you want to get a new one, I think the best deal out there right now on a good oem type all-metal radiator is the CSF sold by autohausaz.com for $163-225 depending on whether you have an automatic that needs the integrated trans fluid cooler. The dealer ones are around $500 for either type - and that's from 1sttoyotaparts.com which gives a huge discount.
When you replace the coolant, be especially sure to use toyota red. If you try prestone green, you'll find your coolant rusty again in a month. The silicates in the green stuff are actually not very good at protecting against iron corrosion. The phosphates in toyota red will do much better. In my truck's case, toyota red lasted a year before it got as rusty as the green stuff did in a month. And of course, flush, flush, flush, flush, flush! It will take a long time before the flush water is free of rust.
(See this post on flushing agents: https://www.yotatech.com/forums/f116.../#post51303314 )
Also be sure if changing coolant types that you get the old stuff out of the plastic overflow tank, and refill up to the proper level with a 50/50 mix of the new coolant.
This info on the different coolant types may be helpful; it's from an earlier post:
RED, GREEN, ORANGE, YELLOW OR PINK? A COOLANT PRIMER
All commonly-available antifreezes are based on ethylene glycol; it's the corrosion inhibitor chemistry that is different and potentially incompatible.
Prior to the late eighties, all coolants supplied in factory pre-fills and available over the counter used conventional inorganic chemistry to inhibit corrosion. Japanese coolants used phosphates, the green stuff (Prestone) used (and still uses) silicates. Both are inorganic chemistries, but they are incompatible with each other and with other coolant types. Phosphates did well with iron, and okay with aluminum, and with copper/brass/lead radiators and heater cores. Silicates (prestone green) are particularly good with aluminum, not as good with iron, and do fine with copper/brass/lead. They are slightly abrasive, and are slightly more likely than phosphates to cause leaks in water pump seals. European carmakers prefer silicates.
There are a couple problems with inorganic corrosion inhibitors. The first is that their ability to buffer (neutralize the acids formed by the breakdown of the ethylene glycol antifreeze) is limited to about 2 years/30k miles. If you allow them to remain in the coolant loop after they have lost their buffering capacity (called "reserve alkalinity"), the coolant will turn acidic and severe corrosion will result. This is what happened with my 3VZE.
The other problem with phosphates and silicates is that mixing with the minerals in tap water or with a different coolant type will cause the phosphates or silicates to precipitate out and clog radiator passages, while at the same time your corrosion protection goes bye-bye.
Because of the problems with straight conventional inorganic corrosion inhibitors, and because of a law passed in Norway in 1987 banning amine, Japanese carmakers were forced to come up with a different coolant. The result was a new coolant type that uses an innovative non-amine anti-corrosion chemistry that combines an organic acid (sodium benzoate) with sodium hydroxide, an inorganic chemical. So, in a sense, these coolants may be considered the first "HOAT"-type coolants: hybrid organic acid technology (more on that later). Toyota Red ("Long Life Coolant") and Subaru's "Long Life Coolant" are two products that use the new chemistry. If you are a geek like me and are interested in the process the Japanese chemical engineers used to develop the new type coolant, here is an interesting in-depth article published in 2002 by Komatsu, a maker of construction equipment: http://www.komatsu.com/CompanyInfo/p...f/149-02_E.pdf
The Japanese Long Life coolants are vastly superior to the old conventional coolants (including Prestone green) at preventing iron corrosion. They also maintain their corrosion protection for significantly longer than conventional coolants, but to be on the safe side, Toyota still recommends changing Toyota Red every two years/30,000 miles. If you run a little over, unlike with Prestone, your engine will not immediately begin to corrode.
Because of their unique chemistry, it is very important never to mix the Japanese "Long Life" coolants with a different coolant type - they will react harmfully, destroying the corrosion protection. You also should never use any coolant additives, because they also will react harmfully with the coolant chemistry. Anyway, there is no need to use additives because Toyota Red is an excellent, well-balanced coolant.
Meanwhile, in the U.S. and Europe, a different type of coolant was developed that was based on organic acids (so-called OAT types), such as Dexcool. (Most OATs are reddish orange.) These are a bad choice for older vehicles because they will corrode copper/brass/lead radiators, and because the OATs react very strongly and harmfully with other coolant types.
In an attempt to address that issue, the antifreeze companies developed a new type called "Hybrid Organic Acid Technology" (HOAT). Those include your Prestone yellow and Zerex G-05 (also yellow). As mentioned, these are similar to Toyota Red (and Subaru Long Life Coolant) in that they combine an organic acid (different from the one used in Dexcool) with inorganic inhibitors. Also, Toyota came up with a new HOAT-type coolant: the new premixed Toyota Pink. That uses sebacic acid, an OAT-type organic acid, rather than the sodium benzoate used in Toyota Red. (Actually Zerex G-05 also uses sodium benzoate, but uses a different inorganic inhibitor.) Confused yet?
Originally, antifreeze makers claimed HOATs could be mixed with any coolant, but those claims have been refuted by evidence that HOATs react badly with OATs. Some of them may mix okay with silicate-based products, but I would bet all HOATs except Toyota Pink would react harmfully with Toyota Red. Basically, you should never mix different coolant types - it's playing with fire.
As far as using HOATs, you have to be careful. Toyota Red is great, but the OAT-type organic acids used in Prestone Yellow and Toyota Pink have the drawback of the OAT coolants: they corrode the lead solder in brass/copper radiators and heater cores. So you don't want to use them in an older vehicle such as ours. Both the OATs and most of the HOATs were designed for aluminum rads & heater cores. It is possible to find aluminum radiators for our trucks, but I've never seen an aluminum replacement heater core.
This August 2004 article in Motor Magazine refers to an industry test of the Toyota pink HOAT coolant that showed "substantial weight loss (corrosion)" of copper-brass radiators with lead solder.
If someone doesn't know what type of coolant is in their radiator, they should FULLY flush every bit of coolant out, making sure to flush the heater core and the plastic overflow reservoir. I recommend removing the thermostat and backflushing the heater core and block with a hose turned to medium flow. When the flush water is clear, drain as much water as possible, then fill the block and radiator with distilled water. You want the last flush to be with distilled because some water always remains behind in the heater core and in other pockets, and it is important not to mix tap water with antifreeze. On my 3VZE, I run the motor with the thermostat removed during this step so I don't have to wait for the water to become hot to open the thermostat and begin circulating.
As far as the proper coolant to use, I recommend what Toyota designed for the motor: Toyota Red coolant. Mix it 50/50 with distilled water. Nothing will provide better corrosion protection to all the different kinds of metal in your coolant loop.
NOTE: As of April 2010, there are reports that Toyota Red may no longer be carried by every dealer. If you can't get it, I see a few alternatives:
First, if Subaru still sells its "Long Life Coolant", P/N SOA868V9210, you're in luck - its ingredients appear to be identical to Toyota Red: Ethylene Glycol, Diethylene glycol, Water, Organic Acid Salt (532-32-1) (which is sodium benzoate), Potassium Hydroxide (1310-58-3) I would still flush well before switching and not mix the two coolants, just to be on the safe side. For one thing, the Subaru Long Life coolant has green dye, a little darker than conventional Prestone's, so mixing it with red would make a mud color which would make it hard to detect rust or other contaminants. The Subaru coolant would be my first choice if Toyota Red were unavailable.
(Dec 2011 edit: another option is Pentosin Pentofrost A1, which the manufacturer claims is chemically identical to Toyota Red - Pentosin may in fact be the OEM source of Toyota's red coolant:
http://www.pentosin.net/pressrelease...frost_A1_3.pdf )
Second, you could use Prestone Green. On the plus side, it is 100% safe for lead/brass/copper radiators and heater cores. On the neg side, it does not protect from iron corrosion nearly as well as Toyota Red and it MUST be changed every 2 years or 30k miles, whichever comes first. Its buffering capacity is limited and it will cause huge corrosion if you leave it in too long. Also it contains silicates, which all Japanese carmakers avoid and don't recommend. They are slightly abrasive and will cause pump seals to fail sooner than with Toyota Red.
(WARNING: Prestone, in their corporate wisdom, now sells an OAT coolant with the organic acid sodium 2-ethyl hexanoate which is Dexcool, with a green dye. It's their "LongLife" product, and comes either in concentrate or premixed. So check the back of any antifreeze you're considering buying. If it contains either of the two most common OAT organic acids (sodium 2-ethyl hexanoate or sebacic acid), DON'T BUY IT. It will corrode the lead solder in your rad/heater core.)
Another possible choice is to use Zerex G-05, because its main inhibitor is the same as Toyota Red's - sodium benzoate. The ingredients of Zerex G-05 are Ethylene Glycol, Diethylene glycol, Sodium Benzoate (532-32-1), Sodium Tetraborate (1330-43-4). So apparently the only difference between that and Toyota Red is the inorganic inhibitor: hydrated potassium hydroxide in Red vs. sodium tetraborate in the G-05. Some forms of borates have been known to cause aluminum pitting, but apparently G-05 is fine for aluminum.
One thing I would NOT do is use Toyota Pink as now suggested by Toyota (grr). That is NOT safe as Toyota's marketing blah blah claims: it will eventually eat through your radiator and heater core, forcing you to replace them.
For the same reason, don't under any circumstances use an OAT coolant like Dexcool because it also will corrode your radiator and heater core.
Replace the thermostat if more than 3-4 years old. The OEM type (a Kuzeh) from the dealer or autohausaz.com is the only one to use - it is far better than any other type. The 3vze stat is a 180 deg, and the 22re stat is 190 degrees. But many 22re folks use a 180 deg stat to fight pinging - that's the t-stat for an 85-88 22RTEC, part # 90916-03083. Be sure to replace the o-ring. On a 3vze, make sure the thermostat's jiggle valve is positioned at the top (12 o'clock). Links to dealers who give online parts discounts:
http://www.toyotanation.com/forum/sh...5&postcount=30
If a 22re runs hotter with the heat on, or the temp gauge spikes up and down, there's a fix for that:
http://www.4crawler.com/4x4/CheapTri...tml#Thermostat
TIPS ON MIXING
I find the easiest way to get a 50/50 mix is to know what the cooling system capacity is and add 50% that much antifreeze, then top off with distilled water. The reason is there is some distilled water left in the system after your flush, but you don't know how much. I've checked my 1989 Pickup fsm and compared it to the 1993 Pickup fsm, and the coolant capacities are identical, though the 93 fsm, as usual, has a couple typos in the converted quarts amounts.
COOLANT CAPACITIES IN 89 AND 93 FSMs - CAPACITIES LISTED ARE FOR PICKUPS WITH HEATER:
22RE NON 4WD A/T - 8.9 US quarts (8.4 liters, 7.4 Imp. qts) Coolant for 50% mix: 4 US qts + 1.75 cups
22RE 4WD A/T models - 9.6 quarts (9.1 liters, 8.0 Imp. qts) Coolant for 50% mix: 4 US qts + 3.25 cups
3VZE 2WD M/T - 11.0 US qts (10.4 liters, 9.2 Imp. qts) Coolant for 50% mix: 5 US qts + 2 cups
3VZE 2WD A/T - 10.8 US qts (10.2 liters, 9.0 Imp. qts) Coolant for 50% mix: 5 US qts + 1.5 cups
3VZE 4WD M/T - 11.1 US qts (10.5 liters, 9.2 Imp. qts) Coolant for 50% mix: 5 US qts + 2.25 cups
3VZE 4WD A/T - 10.9 US qts (10.3 liters, 9.1 Imp. qts) Coolant for 50% mix: 5 US qts + 1.75 cups
Since the 89 & 93 Pickup capacities are identical, probably other years are the same, but to be sure, check the fsm for your year.
COOLANT CAPACITIES FOR 95 4RUNNER:
3VZE M/T - 10.6 US qts (10.0 liters, 8.8 Imp. qts) Coolant for 50% mix: 5 US qts + 1.1 cups
3VZE A/T - 10.4 US qts (9.8 liters, 8.6 Imp. qts) Coolant for 50% mix: 5 US qts + 3/4 cup
FSM capacities are given in liters; all quart amounts are rounded conversions. 50% coolant amounts are half of the capacity amounts in liters, converted to U.S. quarts and cups.
CONSIDERATION: Because Toyota Red, like most antifreezes, is only 97% ethylene glycol, with additives making up the balance, you will need to add a bit more antifreeze than the 50% amounts listed above to reach the -34 freeze point. For 22RE models, the extra amount is about a half cup. For the 3VZE, about 3/4 cup.
You can check your mix with an antifreeze tester/hydrometer. Be sure to use one for ethylene glycol, not propylene glycol. I find the dial type easier to read, and it gives a more exact reading; but the ball/disk type will get you in the ballpark. The freeze point of a 50/50 mix is -34 F, but anywhere between -30 and -40 F will be fine.



FREEZE POINT PER COOLANT PERCENT
AF........FP
%...(˚F).(˚C)
..0....32.....0 (of course)
10....25....-4
15....20....-7
20....15....-9
25....10..-12
30.....5...-15
35....-5...-21
40...-12..-24
45...-23..-31
50...-34..-37
55...-50..-46
60...-65..-54
65...-75..-59
70...-84..-64
75...-70..-57
80...-55..-48
85...-43..-42
90...-30..-34
100...-5..-21
Antifreeze provides freeze and boilover protection, inhibits corrosion, lubricates the water pump and conditions the pump seals. However, antifreeze does not transfer heat as well as water, so the percent of antifreeze you choose is a compromise. More antifreeze provides more freeze and corrosion protection, but at the expense of cooling ability. In most engines, antifreeze percent between 50 and 60 is safe. (Actually, since most antifreeze products contain only 94-97% glycol, with corrosion inhibitors and other additives making up the balance, you may want to use slightly more than 50%.) Up to 70 percent can be used for maximum freeze protection, but only in extreme environments such as near the earth's poles, and then only in winter. In Canada, 55-60 percent is common. Because of the head gasket weakness in the 3VZE, you should not use more than 55 % except during winters where temps remain below freezing.
Corrosion increases a lot below 50% antifreeze, so it's best to use at least that much. BTW, wetting agents like Red Line's "Water Wetter" are not designed for phosphate coolants like Toyota's, and will react to form sludge. Best to avoid them, as you should avoid ALL coolant additives. You don't need them, and they are likely to mess up the coolant's chemistry.
Using a bit more antifreeze will provide a bit more corrosion protection. Because I now have a rusty block, I find my coolant remains clear longer when I use a 55% solution. That also gives me freeze protection all the way down to -50F. But I watch my temp gauge closely in the summer, and if the weather turns too hot, I drain some coolant and replace with distilled water.
TESTING pH - ONE INDICATION THAT THE CORROSION PROTECTION HAS WORN OUT
Another type of test, useful for coolant that has been in use for a while, is pH. As ethylene glycol degrades from heat, it gives off acidic byproducts. These must be neutralized by the coolant, else your engine will begin to corrode. You can get pH test paper from most drug stores - you want the kind that focuses on a narrow range around neutral (7.0). Paper that goes from about 6 to 8 is ideal - but check that the color scale gives you at least a .2 resolution - not all of them do. These strips do not work well in pure glycol, so they are only relevant once the coolant has been mixed with distilled water. The figures here are for Toyota Red coolant - other coolant types have different pH ranges. I've read that the pH range of Prestone Green is 8.5-10.5, but I haven't verified that myself. Also, pH is not the only factor which can indicate worn out coolant - nitrites and chloride levels are also relevant. And ideally, you should measure something called "reserve alkalinity", which is how much buffering capacity the coolant still has. But testing pH is easy and cheap, and helps you at least make sure the coolant is not acidic.
To test pH, quickly dip the strip into the radiator (when not hot), give it one shake to get the excess coolant off, then compare the color to the scale within a half minute or so. If the pH has dropped below 7.1, the coolant has lost much of its buffering capacity and is approaching the end of its useful life. Coolant below 7.0 is acidic and is already corroding your engine - it should be changed immediately. A fresh 50/50 mix of Toyota Red coolant and distilled water has a pH near 7.3 as measured with pH test strips.
Because other factors besides pH can cause a coolant to become corrosive, coolant should be changed at two years, 30k miles, or below 7.0 pH, whichever comes first.
Paper should have at least a .2 resolution:


http://shop.caycecures.com/phtestingpaper.aspx
http://cgi.ebay.com/ws/eBayISAPI.dll...m=110701564252
http://cgi.ebay.com/370384991648
http://www.amazon.com/TheraPure-Heal...dp/B0007RSD0A/
TIPS ON FILLING THE SYSTEM AND GETTING THE AIR OUT
One thing I learned about my veezy is that it's tough to get all the air out of the coolant loop. The heater hoses are higher than the rad, and air gets trapped there (and when filling the radiator, the thermostat stops coolant from entering the engine that way).
Fill the overflow reservoir to the cold level, then elevate the front of the vehicle so the top of the radiator is higher than all the heater hoses, turn heater control all the way to hot to open the heater core, detach the upper rad hose from the radiator and pour coolant/water in the hose to fill the engine, then reattach hose and fill the rad. Keeping the front end elevated and the cap off, run the motor, topping off rad as necessary. If you hear gurgling or water flowing noises in your heater, you know you've still got some air trapped in there.
For the 22re, try filling radiator with the thermostat cover off, but before you install the new thermostat - and with the truck level. When coolant gets to the level of the thermostat, install it with new o-ring, make sure heater control is all the way to hot, then elevate the front of the truck and complete your fill. Then run motor with cap off, adding coolant as necessary.
Original thead is here. The OP mentioned a radiator leak in addition to the rusty coolant.
--------------------------------------------------------------------------------------------------
Rusty coolant means coolant that lost its corrosion protection and became acidic. This will happen if not changed every two years, or if non-distilled water was used, or if different types of antifreeze were mixed (either by adding a different type or by not flushing thoroughly, including the heater core and plastic overflow tank), or by using one of the organic acid-based extended life coolants.
That's why the leak in the radiator.
The problem is that the corrosion is not limited to the radiator. The rust indicates the block has corroded, and, no doubt, the steel sealing rings on your head gasket(s) also. You'll have to keep a close eye out for signs of a failed head gasket.
I know because I made the same mistake with the same result except my pinhole leak was in one of the coolant pipes in that spaghetti cluster of vacuum and coolant pipes bolted to the front of the plenum on a 3vze. And not long after, my head gasket failed on cyl #1. When I removed the heads, I found the sealing ring there completely corroded with rust caused by the adjacent coolant gallery.
In my case I had replaced the toyota red coolant with prestone green, without flushing as thoroughly as I needed to, thus mixing two incompatible coolants which resulted in some of the silicates in the incoming coolant being neutralized by the phosphates in the old coolant. I think I also probably used tap water, further degrading the corrosion protection in the new coolant. A year later, like you, I opened the radiator cap one day to find the coolant completely rusty.
If you can let us know what type of coolant you used, how long it has been in there, whether it was mixed with any other type, and whether tap water was used, that will be useful info for the other folks who will read this.
I suppose its worth a try to patch your radiator, but if you want to get a new one, I think the best deal out there right now on a good oem type all-metal radiator is the CSF sold by autohausaz.com for $163-225 depending on whether you have an automatic that needs the integrated trans fluid cooler. The dealer ones are around $500 for either type - and that's from 1sttoyotaparts.com which gives a huge discount.
When you replace the coolant, be especially sure to use toyota red. If you try prestone green, you'll find your coolant rusty again in a month. The silicates in the green stuff are actually not very good at protecting against iron corrosion. The phosphates in toyota red will do much better. In my truck's case, toyota red lasted a year before it got as rusty as the green stuff did in a month. And of course, flush, flush, flush, flush, flush! It will take a long time before the flush water is free of rust.
(See this post on flushing agents: https://www.yotatech.com/forums/f116.../#post51303314 )
Also be sure if changing coolant types that you get the old stuff out of the plastic overflow tank, and refill up to the proper level with a 50/50 mix of the new coolant.
This info on the different coolant types may be helpful; it's from an earlier post:
RED, GREEN, ORANGE, YELLOW OR PINK? A COOLANT PRIMER
All commonly-available antifreezes are based on ethylene glycol; it's the corrosion inhibitor chemistry that is different and potentially incompatible.
Prior to the late eighties, all coolants supplied in factory pre-fills and available over the counter used conventional inorganic chemistry to inhibit corrosion. Japanese coolants used phosphates, the green stuff (Prestone) used (and still uses) silicates. Both are inorganic chemistries, but they are incompatible with each other and with other coolant types. Phosphates did well with iron, and okay with aluminum, and with copper/brass/lead radiators and heater cores. Silicates (prestone green) are particularly good with aluminum, not as good with iron, and do fine with copper/brass/lead. They are slightly abrasive, and are slightly more likely than phosphates to cause leaks in water pump seals. European carmakers prefer silicates.
There are a couple problems with inorganic corrosion inhibitors. The first is that their ability to buffer (neutralize the acids formed by the breakdown of the ethylene glycol antifreeze) is limited to about 2 years/30k miles. If you allow them to remain in the coolant loop after they have lost their buffering capacity (called "reserve alkalinity"), the coolant will turn acidic and severe corrosion will result. This is what happened with my 3VZE.
The other problem with phosphates and silicates is that mixing with the minerals in tap water or with a different coolant type will cause the phosphates or silicates to precipitate out and clog radiator passages, while at the same time your corrosion protection goes bye-bye.
Because of the problems with straight conventional inorganic corrosion inhibitors, and because of a law passed in Norway in 1987 banning amine, Japanese carmakers were forced to come up with a different coolant. The result was a new coolant type that uses an innovative non-amine anti-corrosion chemistry that combines an organic acid (sodium benzoate) with sodium hydroxide, an inorganic chemical. So, in a sense, these coolants may be considered the first "HOAT"-type coolants: hybrid organic acid technology (more on that later). Toyota Red ("Long Life Coolant") and Subaru's "Long Life Coolant" are two products that use the new chemistry. If you are a geek like me and are interested in the process the Japanese chemical engineers used to develop the new type coolant, here is an interesting in-depth article published in 2002 by Komatsu, a maker of construction equipment: http://www.komatsu.com/CompanyInfo/p...f/149-02_E.pdf
The Japanese Long Life coolants are vastly superior to the old conventional coolants (including Prestone green) at preventing iron corrosion. They also maintain their corrosion protection for significantly longer than conventional coolants, but to be on the safe side, Toyota still recommends changing Toyota Red every two years/30,000 miles. If you run a little over, unlike with Prestone, your engine will not immediately begin to corrode.
Because of their unique chemistry, it is very important never to mix the Japanese "Long Life" coolants with a different coolant type - they will react harmfully, destroying the corrosion protection. You also should never use any coolant additives, because they also will react harmfully with the coolant chemistry. Anyway, there is no need to use additives because Toyota Red is an excellent, well-balanced coolant.
Meanwhile, in the U.S. and Europe, a different type of coolant was developed that was based on organic acids (so-called OAT types), such as Dexcool. (Most OATs are reddish orange.) These are a bad choice for older vehicles because they will corrode copper/brass/lead radiators, and because the OATs react very strongly and harmfully with other coolant types.
In an attempt to address that issue, the antifreeze companies developed a new type called "Hybrid Organic Acid Technology" (HOAT). Those include your Prestone yellow and Zerex G-05 (also yellow). As mentioned, these are similar to Toyota Red (and Subaru Long Life Coolant) in that they combine an organic acid (different from the one used in Dexcool) with inorganic inhibitors. Also, Toyota came up with a new HOAT-type coolant: the new premixed Toyota Pink. That uses sebacic acid, an OAT-type organic acid, rather than the sodium benzoate used in Toyota Red. (Actually Zerex G-05 also uses sodium benzoate, but uses a different inorganic inhibitor.) Confused yet?
Originally, antifreeze makers claimed HOATs could be mixed with any coolant, but those claims have been refuted by evidence that HOATs react badly with OATs. Some of them may mix okay with silicate-based products, but I would bet all HOATs except Toyota Pink would react harmfully with Toyota Red. Basically, you should never mix different coolant types - it's playing with fire.
As far as using HOATs, you have to be careful. Toyota Red is great, but the OAT-type organic acids used in Prestone Yellow and Toyota Pink have the drawback of the OAT coolants: they corrode the lead solder in brass/copper radiators and heater cores. So you don't want to use them in an older vehicle such as ours. Both the OATs and most of the HOATs were designed for aluminum rads & heater cores. It is possible to find aluminum radiators for our trucks, but I've never seen an aluminum replacement heater core.
This August 2004 article in Motor Magazine refers to an industry test of the Toyota pink HOAT coolant that showed "substantial weight loss (corrosion)" of copper-brass radiators with lead solder.
Results of industry standard tests of the new Toyota extended-life coolant now show a substantial weight loss (corrosion), both in a 50-50 mix and in a 33% coolant mixture (solder corrosion is much greater in this more diluted solution).
As far as the proper coolant to use, I recommend what Toyota designed for the motor: Toyota Red coolant. Mix it 50/50 with distilled water. Nothing will provide better corrosion protection to all the different kinds of metal in your coolant loop.
NOTE: As of April 2010, there are reports that Toyota Red may no longer be carried by every dealer. If you can't get it, I see a few alternatives:
First, if Subaru still sells its "Long Life Coolant", P/N SOA868V9210, you're in luck - its ingredients appear to be identical to Toyota Red: Ethylene Glycol, Diethylene glycol, Water, Organic Acid Salt (532-32-1) (which is sodium benzoate), Potassium Hydroxide (1310-58-3) I would still flush well before switching and not mix the two coolants, just to be on the safe side. For one thing, the Subaru Long Life coolant has green dye, a little darker than conventional Prestone's, so mixing it with red would make a mud color which would make it hard to detect rust or other contaminants. The Subaru coolant would be my first choice if Toyota Red were unavailable.
(Dec 2011 edit: another option is Pentosin Pentofrost A1, which the manufacturer claims is chemically identical to Toyota Red - Pentosin may in fact be the OEM source of Toyota's red coolant:
http://www.pentosin.net/pressrelease...frost_A1_3.pdf )
Second, you could use Prestone Green. On the plus side, it is 100% safe for lead/brass/copper radiators and heater cores. On the neg side, it does not protect from iron corrosion nearly as well as Toyota Red and it MUST be changed every 2 years or 30k miles, whichever comes first. Its buffering capacity is limited and it will cause huge corrosion if you leave it in too long. Also it contains silicates, which all Japanese carmakers avoid and don't recommend. They are slightly abrasive and will cause pump seals to fail sooner than with Toyota Red.
(WARNING: Prestone, in their corporate wisdom, now sells an OAT coolant with the organic acid sodium 2-ethyl hexanoate which is Dexcool, with a green dye. It's their "LongLife" product, and comes either in concentrate or premixed. So check the back of any antifreeze you're considering buying. If it contains either of the two most common OAT organic acids (sodium 2-ethyl hexanoate or sebacic acid), DON'T BUY IT. It will corrode the lead solder in your rad/heater core.)
Another possible choice is to use Zerex G-05, because its main inhibitor is the same as Toyota Red's - sodium benzoate. The ingredients of Zerex G-05 are Ethylene Glycol, Diethylene glycol, Sodium Benzoate (532-32-1), Sodium Tetraborate (1330-43-4). So apparently the only difference between that and Toyota Red is the inorganic inhibitor: hydrated potassium hydroxide in Red vs. sodium tetraborate in the G-05. Some forms of borates have been known to cause aluminum pitting, but apparently G-05 is fine for aluminum.
One thing I would NOT do is use Toyota Pink as now suggested by Toyota (grr). That is NOT safe as Toyota's marketing blah blah claims: it will eventually eat through your radiator and heater core, forcing you to replace them.
For the same reason, don't under any circumstances use an OAT coolant like Dexcool because it also will corrode your radiator and heater core.
Replace the thermostat if more than 3-4 years old. The OEM type (a Kuzeh) from the dealer or autohausaz.com is the only one to use - it is far better than any other type. The 3vze stat is a 180 deg, and the 22re stat is 190 degrees. But many 22re folks use a 180 deg stat to fight pinging - that's the t-stat for an 85-88 22RTEC, part # 90916-03083. Be sure to replace the o-ring. On a 3vze, make sure the thermostat's jiggle valve is positioned at the top (12 o'clock). Links to dealers who give online parts discounts:
http://www.toyotanation.com/forum/sh...5&postcount=30
If a 22re runs hotter with the heat on, or the temp gauge spikes up and down, there's a fix for that:
http://www.4crawler.com/4x4/CheapTri...tml#Thermostat
TIPS ON MIXING
I find the easiest way to get a 50/50 mix is to know what the cooling system capacity is and add 50% that much antifreeze, then top off with distilled water. The reason is there is some distilled water left in the system after your flush, but you don't know how much. I've checked my 1989 Pickup fsm and compared it to the 1993 Pickup fsm, and the coolant capacities are identical, though the 93 fsm, as usual, has a couple typos in the converted quarts amounts.
COOLANT CAPACITIES IN 89 AND 93 FSMs - CAPACITIES LISTED ARE FOR PICKUPS WITH HEATER:
22RE NON 4WD A/T - 8.9 US quarts (8.4 liters, 7.4 Imp. qts) Coolant for 50% mix: 4 US qts + 1.75 cups
22RE 4WD A/T models - 9.6 quarts (9.1 liters, 8.0 Imp. qts) Coolant for 50% mix: 4 US qts + 3.25 cups
3VZE 2WD M/T - 11.0 US qts (10.4 liters, 9.2 Imp. qts) Coolant for 50% mix: 5 US qts + 2 cups
3VZE 2WD A/T - 10.8 US qts (10.2 liters, 9.0 Imp. qts) Coolant for 50% mix: 5 US qts + 1.5 cups
3VZE 4WD M/T - 11.1 US qts (10.5 liters, 9.2 Imp. qts) Coolant for 50% mix: 5 US qts + 2.25 cups
3VZE 4WD A/T - 10.9 US qts (10.3 liters, 9.1 Imp. qts) Coolant for 50% mix: 5 US qts + 1.75 cups
Since the 89 & 93 Pickup capacities are identical, probably other years are the same, but to be sure, check the fsm for your year.
COOLANT CAPACITIES FOR 95 4RUNNER:
3VZE M/T - 10.6 US qts (10.0 liters, 8.8 Imp. qts) Coolant for 50% mix: 5 US qts + 1.1 cups
3VZE A/T - 10.4 US qts (9.8 liters, 8.6 Imp. qts) Coolant for 50% mix: 5 US qts + 3/4 cup
FSM capacities are given in liters; all quart amounts are rounded conversions. 50% coolant amounts are half of the capacity amounts in liters, converted to U.S. quarts and cups.
CONSIDERATION: Because Toyota Red, like most antifreezes, is only 97% ethylene glycol, with additives making up the balance, you will need to add a bit more antifreeze than the 50% amounts listed above to reach the -34 freeze point. For 22RE models, the extra amount is about a half cup. For the 3VZE, about 3/4 cup.
You can check your mix with an antifreeze tester/hydrometer. Be sure to use one for ethylene glycol, not propylene glycol. I find the dial type easier to read, and it gives a more exact reading; but the ball/disk type will get you in the ballpark. The freeze point of a 50/50 mix is -34 F, but anywhere between -30 and -40 F will be fine.



FREEZE POINT PER COOLANT PERCENT
AF........FP
%...(˚F).(˚C)
..0....32.....0 (of course)
10....25....-4
15....20....-7
20....15....-9
25....10..-12
30.....5...-15
35....-5...-21
40...-12..-24
45...-23..-31
50...-34..-37
55...-50..-46
60...-65..-54
65...-75..-59
70...-84..-64
75...-70..-57
80...-55..-48
85...-43..-42
90...-30..-34
100...-5..-21
Antifreeze provides freeze and boilover protection, inhibits corrosion, lubricates the water pump and conditions the pump seals. However, antifreeze does not transfer heat as well as water, so the percent of antifreeze you choose is a compromise. More antifreeze provides more freeze and corrosion protection, but at the expense of cooling ability. In most engines, antifreeze percent between 50 and 60 is safe. (Actually, since most antifreeze products contain only 94-97% glycol, with corrosion inhibitors and other additives making up the balance, you may want to use slightly more than 50%.) Up to 70 percent can be used for maximum freeze protection, but only in extreme environments such as near the earth's poles, and then only in winter. In Canada, 55-60 percent is common. Because of the head gasket weakness in the 3VZE, you should not use more than 55 % except during winters where temps remain below freezing.
Corrosion increases a lot below 50% antifreeze, so it's best to use at least that much. BTW, wetting agents like Red Line's "Water Wetter" are not designed for phosphate coolants like Toyota's, and will react to form sludge. Best to avoid them, as you should avoid ALL coolant additives. You don't need them, and they are likely to mess up the coolant's chemistry.
Using a bit more antifreeze will provide a bit more corrosion protection. Because I now have a rusty block, I find my coolant remains clear longer when I use a 55% solution. That also gives me freeze protection all the way down to -50F. But I watch my temp gauge closely in the summer, and if the weather turns too hot, I drain some coolant and replace with distilled water.
TESTING pH - ONE INDICATION THAT THE CORROSION PROTECTION HAS WORN OUT
Another type of test, useful for coolant that has been in use for a while, is pH. As ethylene glycol degrades from heat, it gives off acidic byproducts. These must be neutralized by the coolant, else your engine will begin to corrode. You can get pH test paper from most drug stores - you want the kind that focuses on a narrow range around neutral (7.0). Paper that goes from about 6 to 8 is ideal - but check that the color scale gives you at least a .2 resolution - not all of them do. These strips do not work well in pure glycol, so they are only relevant once the coolant has been mixed with distilled water. The figures here are for Toyota Red coolant - other coolant types have different pH ranges. I've read that the pH range of Prestone Green is 8.5-10.5, but I haven't verified that myself. Also, pH is not the only factor which can indicate worn out coolant - nitrites and chloride levels are also relevant. And ideally, you should measure something called "reserve alkalinity", which is how much buffering capacity the coolant still has. But testing pH is easy and cheap, and helps you at least make sure the coolant is not acidic.
To test pH, quickly dip the strip into the radiator (when not hot), give it one shake to get the excess coolant off, then compare the color to the scale within a half minute or so. If the pH has dropped below 7.1, the coolant has lost much of its buffering capacity and is approaching the end of its useful life. Coolant below 7.0 is acidic and is already corroding your engine - it should be changed immediately. A fresh 50/50 mix of Toyota Red coolant and distilled water has a pH near 7.3 as measured with pH test strips.
Because other factors besides pH can cause a coolant to become corrosive, coolant should be changed at two years, 30k miles, or below 7.0 pH, whichever comes first.
Paper should have at least a .2 resolution:

http://shop.caycecures.com/phtestingpaper.aspx
http://cgi.ebay.com/ws/eBayISAPI.dll...m=110701564252
http://cgi.ebay.com/370384991648
http://www.amazon.com/TheraPure-Heal...dp/B0007RSD0A/
TIPS ON FILLING THE SYSTEM AND GETTING THE AIR OUT
One thing I learned about my veezy is that it's tough to get all the air out of the coolant loop. The heater hoses are higher than the rad, and air gets trapped there (and when filling the radiator, the thermostat stops coolant from entering the engine that way).
Fill the overflow reservoir to the cold level, then elevate the front of the vehicle so the top of the radiator is higher than all the heater hoses, turn heater control all the way to hot to open the heater core, detach the upper rad hose from the radiator and pour coolant/water in the hose to fill the engine, then reattach hose and fill the rad. Keeping the front end elevated and the cap off, run the motor, topping off rad as necessary. If you hear gurgling or water flowing noises in your heater, you know you've still got some air trapped in there.
For the 22re, try filling radiator with the thermostat cover off, but before you install the new thermostat - and with the truck level. When coolant gets to the level of the thermostat, install it with new o-ring, make sure heater control is all the way to hot, then elevate the front of the truck and complete your fill. Then run motor with cap off, adding coolant as necessary.
Last edited by sb5walker; 12-03-2011 at 02:22 PM.
#4
Registered User
I'm on fence concerning old green vs. Toyota Red, but they take different approaches. More is involved than color. I found this:

Last edited by flyingbrass; 12-02-2009 at 09:16 PM.
#5
Registered User
Thread Starter
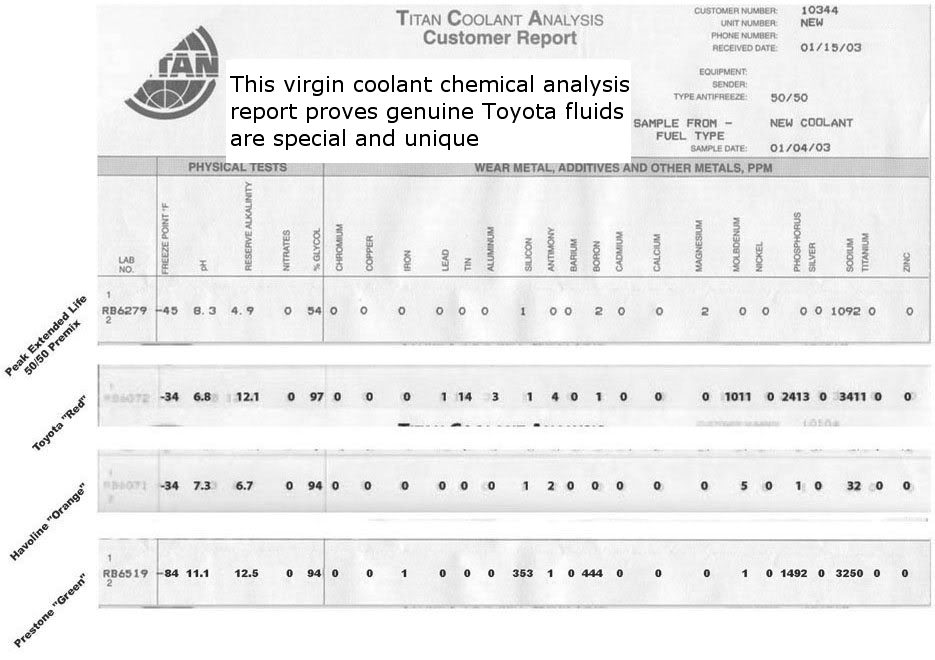
A 50/50 mix of fresh toyota red & distilled water has a pH of 7.3, so I wonder if they mixed up some of the numbers between the red and the Havoline?
#6
Registered User
Join Date: Oct 2006
Location: Greeley Colorado
Posts: 746
Likes: 0
Received 0 Likes
on
0 Posts
ph - power of hydrogen- your rad fluid should be about 9 ph, every .1 on ph is 10 times aluminmum needs this side of the ph scale due to the temps, temp and ph goes hand and hand
#7
Contributing Member
Join Date: Jun 2007
Location: Lake County, CA/Sacramento
Posts: 4,222
Likes: 0
Received 3 Likes
on
3 Posts
From that chart, it looks like the Havoline Orange would potentially be the least corrosive, due to the mutually agreed reasoning that you want to have a relatively stable pH range, with little fluctuation.
Interesting that the Havoline Orange also has an EXTREMELY small portion of Sodium compared to the others.
This being said, I seriously doubt, me switching to either of these will make any difference at all(other than the size of the dent in my wallet) at 200,000+ miles.
Very interesting read, though, thanks for sharing!!!
Interesting that the Havoline Orange also has an EXTREMELY small portion of Sodium compared to the others.
This being said, I seriously doubt, me switching to either of these will make any difference at all(other than the size of the dent in my wallet) at 200,000+ miles.
Very interesting read, though, thanks for sharing!!!
Trending Topics
#8
Registered User
Thread Starter
Well I can't say I understand the point but I will say that different coolant types have different pH ranges. 9 is in the range for Prestone green, not Toyota Red which is a mild base at just above neutral (7). Corrosion actually increases in higher alkaline environments, not just in lower acidic ones. Most modern coolants have pH ranges near that of Toyota Red.
From that chart, it looks like the Havoline Orange would potentially be the least corrosive, due to the mutually agreed reasoning that you want to have a relatively stable pH range, with little fluctuation.
Interesting that the Havoline Orange also has an EXTREMELY small portion of Sodium compared to the others.
Interesting that the Havoline Orange also has an EXTREMELY small portion of Sodium compared to the others.
Sodium in antifreeze comes from various metal salts used as corrosion inhibitors. Sodium silicate being the main one in Prestone Green, and Sodium Borate and Sodium Molybdate are two others sometimes used in conventional and HOAT coolants. OATs and HOATs use organic acid salts as their main inhibitors, though, as mentioned, HOATs also add some inorganic salts, usually sodium silicate. Toyota Red uses sodium benzoate, an organic acid salt, as the primary corrosion inhibitor.
The chart appears to have a number of contradictions, so I don't know whether anything can safely be concluded from it. At the top it says it's a virgin coolant analysis and for new coolant. So it would not have any wear metals in it to show how much corrosion occurred with any of them.
All these coolants use ethylene glycol as the antifreeze, so the freeze point should reflect the glycol percent. Yet the freeze points shown for every sample are wrong based on the glycol percent - not one of them is correct. The pH of the Toyota Red sample is wrong, but as mentioned in the opening post that is probably because conventional pH testing doesn't work in pure glycol - you have to mix it with water to get an approximately accurate reading.
Last edited by sb5walker; 11-23-2010 at 06:20 PM.
#9
Excellent write up on the different types of coolant.
Let me show you what happens when someone, in my case the PO, doesn't change and/or uses the cheap stuff.
This is what I drained out of my radiator today.
No... that isn't chocolate milk!!

Let me show you what happens when someone, in my case the PO, doesn't change and/or uses the cheap stuff.
This is what I drained out of my radiator today.
No... that isn't chocolate milk!!

#10
Registered User
Join Date: Nov 2010
Location: I'm a Masshole
Posts: 129
Likes: 0
Received 0 Likes
on
0 Posts
I know this is bumping an old thread, but I wanted to post a piece of useful information I came across.
I had my coolant flushed out this weekend at the local Subaru dealer, and asked for their long life coolant and on the line items, the coolant was the Subaru Long Life Coolant, but in quart containers. Because of this, the part number is different.
Per the above thread, for the gallon container, the coolant part number is: SOA868V9210
The part number for the quart containers is:
SOA635002
I don't know if one is available and one isn't, but this is what they used on my truck this past weekend. I have a Subaru also, so I just brought it in and requested it while they were running a special.
I had my coolant flushed out this weekend at the local Subaru dealer, and asked for their long life coolant and on the line items, the coolant was the Subaru Long Life Coolant, but in quart containers. Because of this, the part number is different.
Per the above thread, for the gallon container, the coolant part number is: SOA868V9210
The part number for the quart containers is:
SOA635002
I don't know if one is available and one isn't, but this is what they used on my truck this past weekend. I have a Subaru also, so I just brought it in and requested it while they were running a special.
#11
Registered User
iTrader: (1)
Another possibility is Amsoil propylene glycol coolant. It's expensive, but tests show that it is very gentle on all kinds of metals. I will probably switch back to it once I get all the various leaks in my coolant system worked out! For now I am running Prestone, but I also installed a coolant filter in parallel with the heater circuit to catch any corroded bits.
Thread
Thread Starter
Forum
Replies
Last Post
jasonty
Pre 84 Trucks (Build-Up Section)
41
12-23-2018 01:00 PM
hiluxinargentina
86-95 Trucks & 4Runners
0
09-30-2015 11:12 PM